Impulse heat sealers with Sealing Parameters total Control.
Validatable Sealing Process according to ISO 11607.2, ISO TS 16775, EN 868-2, DIN 58953-7 Standards.
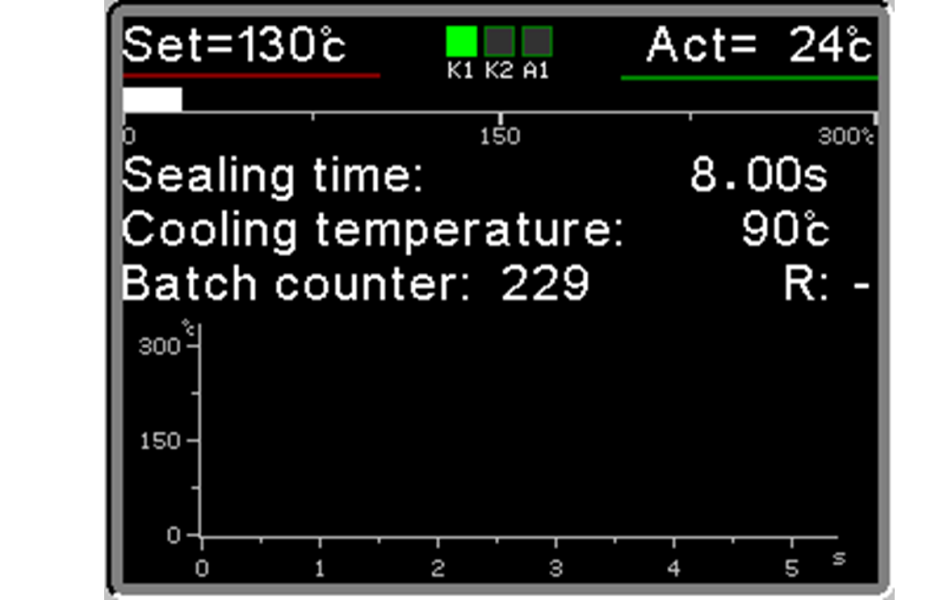
Today equipped as standard with the new Ropex RESISTRON® RES 5450 controller, even more performing.
AISI 304 stainless steel cover.
Impulse sealing, flat, sealing height mm 8, max sealing lengths mm 460/610/710.
Heat-sealable materials: sealable sterile barrier systems, Tyvek®, new generation materials.
Manual operation or via electric foot switch (optional).
Advantages and Benefits
Packaging Process Validation
Packaging Process Validation
High technology for extremely accurate control of sealing parameters to guarantee perfect compliance with ISO 11607.2 standards and international guidelines EN-ISO TS 16775. The three sealing parameters, Temperature – Force – Time, are constantly monitored 50 times per second by the new Ropex RESISTRON® RES 5450 controller, thus ensuring absolute consistency and quality of sealing. If one of the parameters exceeds the set tolerance thresholds, the machine stops and the alarm signal appears on the display.
Safety
mm 8 flat seal for perfect seal and effective peelability.
Communication interfaces
Interface via USB-C port to communicate with dedicated traceability software.
Traceability
Remote Management Software, dedicated traceability software that can be interfaced with a Personal Computer to remotely set the sealing machine and record and manage operating data to ensure traceability of sealing parameters and documentation of process Validation.
Operator Interface
LCD interface and easy-to-use software for a very ergonomic approach to the sealer.
Sanitation
The stainless steel cover allows for maximum cleaning and disinfection.
Materials
Designed for sealing all sealable sterile barrier systems, Tyvek®, all new generation packaging materials.
Versions
Sealing lengths mm 460 – mm 610 – mm 710 – mm 810
Standard Version and GT Version, with built-in roll holder and cutter. Cutting length mm 370/500/610/610.
Accessories
Work plan
PR H40 roll holder complete with cutting device
PR H50 roll holder complete with cutting device
Thermometer and probe kit for temperature calibration
Compliance
UNI EN ISO 11607-2
UNI EN 868-5
DIN-58953 7
2006/42/EC-2014/30/UE-2014/35/UE
UNI/TR 11408
Produced in a Company certified with the UNI EN ISO 9001:2015 Quality System
Testing and Validation Services
Gandus Saldatrici offers a range of Testing and Validation Services to ensure safe and reliable daily monitoring of medical sealing machines for sterilization pouches in accordance with ISO 11607.1.2 and ASTM F1929 Standards. learn more