Validatable Pneumatic heat sealers according to ISO 11607.2, ISO TS 16775, EN 868-2, DIN 58953-7 standards.
Vertical or horizontal sealing depending on whether or not the support is used.
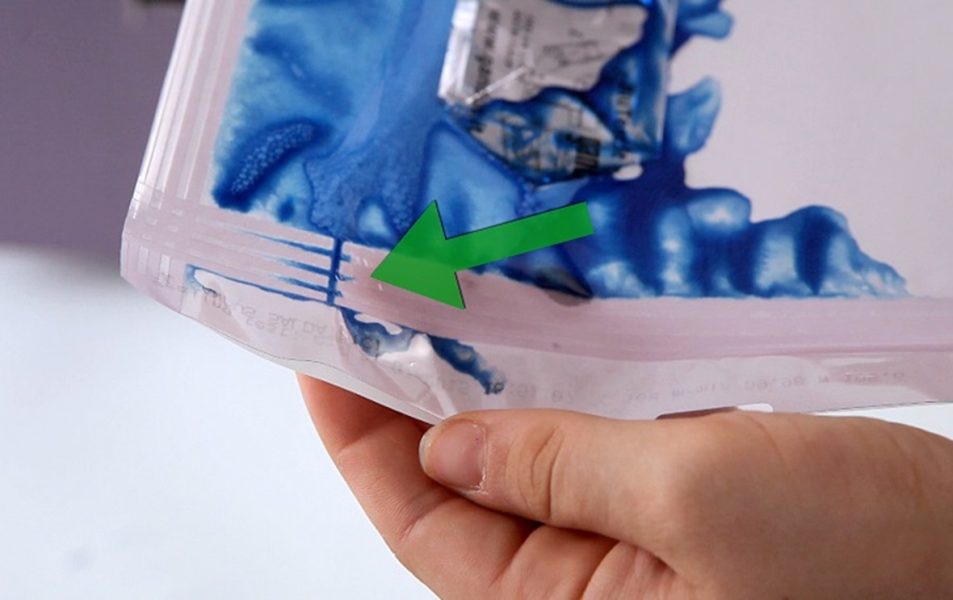
mm 8 flat sealing.
Sealing length mm 720.
Heat sealable materials: EN 868-5 pouches/rolls, Tyvek®, EN 868-4 paper/paper, with gussets, Laminated aluminium, PP, Header bags, Polyethylene, Ultra®
Advantages and Benefits
Packaging Process Validation
Automatic control and recording of critical sealing parameters to ensure constant and repeatable high quality over time, compliant with ISO 11607.2 Standards and International Guidelines EN-ISO TS 16775.
Safety
mm 8 flat seal for a perfect seal and effective peelability.
Communication interfaces
DIAG port
Interface with PC
Traceability
Traceability of sealing parameters and documentation of Process Validation.
Operator Interface
Colour LCD
Membrane keyboard
Sanitation
Stainless steel for maximum cleaning and disinfection.
Applications
Designed specifically for the medical, pharmaceutical, laboratory and chemical industries
Materials
Bags/Rolls EN 868-5, Tyvek®, Paper/Paper EN 868-4, with gussets, Laminated Aluminium, PP, Header bags, Polyethylene, Ultra®
Versions
Length mm 520, mm 720, mm 1020
Vacuum version per sottovuoto (SI Vacuum(Med.)
IP 65 version
Accessories
SIS height-adjustable support, allows vertical sealing
SIPI inclined work table (only with SIS)
SIPO horizontal work table (only with SIS)
RL 200 Independent conveyor belt 2000 x 500mm (only with SIS)
Compliance
ISO 11607.2
ISO TS 16775
Directive EN 868-5
Directive EN 868-2
DIN-58953 7
Produced in a Company certified with the UNI EN ISO 9001:2015 Quality System
Testing and Validation Services
Gandus Saldatrici offers a range of Testing and Validation Services to ensure safe and reliable daily monitoring of medical sealing machines for sterilization pouches in accordance with ISO 11607.1.2 and ASTM F1929 Standards. learn more